Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006, concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals, Official Journal No. L 396/1 of 30.12.2006 (hereinafter “REACH”) aims at ensuring a high level of protection for human health and environment, while promoting the efficient functioning of the EU internal market and stimulating innovation and competitiveness in the chemical industry.
REACH provides for requirements on manufacturers and importers of substances, concerning more specifically the registration of substances on their own, in preparations or in articles.
REACH affects directly or indirectly manufacturers and importers established within or outside the EU.
REACH provides for, amongst others, an obligation to share vertebrate animal studies, and to jointly submit part of the registration. Considering the human and financial resources required by registration, together with the limited time to ensure compliance and to remain compliant, it is necessary to increase and/or improve efficiency of the workload as well as cost-efficiency.
Having a common interest in fulfilling the requirements under REACH, the members of the As Consortium have therefore created the As Consortium back in 2009, in order to share human and financial resources involved in complying with REACH.
The following substances were REACH registered with the help of the As consortium:
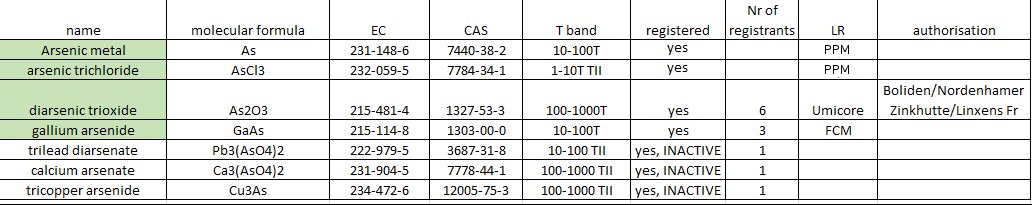
A Letter of Access (LoA) is available for As2O3, GaAs, AsCl3 and As metal . For more information: please contact the Secretary General.
More information on the ECHA dissemination website:
For GaAs: https://echa.europa.eu/
For As2O3: https://echa.europa.eu/
For As: https://echa.europa.eu/nl/